Inventive step was denied because new knowledge was not concretely described in specification of claimed invention.
IP Court Case Summary:H23 (gyoke) 10091:
May 7, 2012, the Intellectual Property High Court (IP High Court) reversed JPO board of appeal’s decision on the trial for invalidation of the patent. (Inventive step are discussed here.)
The title of the invention at issue is “Stable oral CI-981 formulation and process of preparing same”. Claim-1 is “A pharmaceutical composition for the peroral treatment of hypercholesterolemia or hyperlipidemia characterized by improved stability comprising in a mixture, a compound as active ingredient of [R(R ,R*)]-2-(4-fluorophenyl-ss,6-dihydroxy-5(1-methylethyl)-3-phenyl-4-[(phenylamino)- carbonyl]-lH-pyrrole-1-heptanoic acid Hemi Calcium and at least one stabilizing pharmaceutically acceptable basic metal salt additive”.
(“[R(R ,R*)]-2-(4-fluorophenyl-ss,6-dihydroxy-5(1-methylethyl)-3-phenyl-4-[(phenylamino)- carbonyl]-lH-pyrrole-1-heptanoic acid” is referred to “CI-981”.)
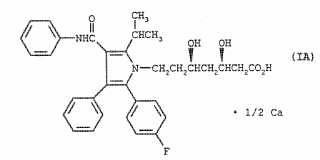
[CI-981 Hemi Calcium]
During the trial for invalidation by JPO board of appeal, the plaintiff insisted that a person ordinarily skilled in the art could easily invent claimed invention (Claim-1) based on citation-1 and citation-2. However, JPO board of appeal finally approved the inventive step of claim-1.
Claim-1 applied “CI-981 Hemi Calcium” as “HMG-CoA reductase enzyme inhibitors” which is active ingredient of “pharmaceutical composition for the peroral treatment of hypercholesterolemia or hyperlipidemia”. Citation-1 applied “Pravastatin” as “HMG-CoA reductase enzyme inhibitors” in “pharmaceutical composition for the peroral treatment of hypercholesterolemia or hyperlipidemia”. Therefore, the difference between claim-1 and citation-1 is “CI-981 Hemi Calcium” or “Pravastatin”. On the other hand, citation-2 described “CI-981” as “HMG-CoA reductase enzyme inhibitors”, and explained that “CI-981” can be applied as lactone compounds and “CI-981 Hemi Calcium” is the most preferable form.
JPO board of appeal approved the inventive step of claime-1 based on the assumption that “CI-981 Hemi Calcium is more effective than lactone compounds, which was found by claimed invention.” However, IP High Court rejected the assumption by JPO board of appeal because the specification of claim-1 has only a simple sentence of “CI-981 Hemi Calcium is the most preferable.” and does not concretely explain the advantageous effect of CI-981 Hemi Calcium in comparison with lactone compounds.
IP High Court finally reversed JPO board of appeal’s decision, and denied the inventive step of Claim-1.
<Comments>
If certain knowledge was not supported in a citation even if there is simple description about the certain knowledge in the citation, this citation may not be adopted as proof to deny patentability (novelty, inventive step). Typically, as for the compound which was merely described multiply and was not described specifically in a citation, this citation may not be adopted as proof to deny patentability. However in this case, this precedent suggested that this knowledge should be supported in the specification of claimed invention. Thus, not only the fact that the knowledge is NOT supported in citation, but also that the knowledge is fully supported in specification of claimed invention is necessary for inventive step.
http://www.ip.courts.go.jp/hanrei/pdf/20120508162813.pdf

