IP High Court Case (en banc) Summary:2017 (Ne) 10014:
Patent right was infringed by the reason for the equivalency to the claimed invention in Pharmaceutical field.
March 25, 2016, the Intellectual Property High Court (IP High Court) determined the infringement by the reason for the equivalency to the claimed invention in Pharmaceutical field. Then, District Court decision was affirmed.
<Outline of this case>
The title of the patented invention at issue is “Intermediates for the Synthesis of Vitamin D and Steroid Derivatives and Processes for Preparation thereof”. Chugai Pharmaceutical Co., Ltd. who has this patent asserted that the generic companies infringed this patent right because the medicines of the generic companies are equivalent to the claimed invention of this patent. Then, Chugai demand the injunction of the medicines of the generic companies.
District Court affirmed this equivalency and the validity of this patent. The generic companies were not satisfied with this decision. Then, it was appealed to IP High Court.
<Claimed invention and medicines of the generic companies>
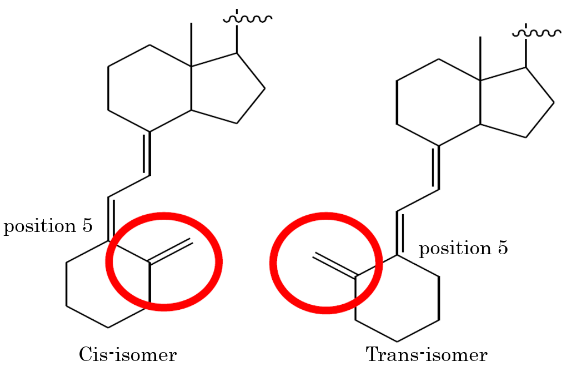
Claimed invention of this patent is related to the process for manufacturing the targeted chemical substance (Maxacalcitol). Medicines of the generic companies are the same as the most parts of this claimed invention, but the structure of Vitamin D is trans-isomer while that of claimed invention is cis-isomer.
<Decision of IP High Court>
IP High Court affirmed the equivalency to the claimed invention and the validity of this patent. Then, the decision of Tokyo District Court was affirmed.
As for the equivalency, Supreme Court Decision “Ballspline case” (February 24, 1998; H6 (O) 1083) suggested five requirements for the equivalency (see Note at the end of this document). In this case, all the five requirements were discussed. Here, the first requirement and the fifth requirement are focused.
(1) First requirement for the equivalency (Non-essential elements)
First requirement for the equivalency under the “Ballspline case” requires that the differences between the claimed inventions and the products at issue are not the essential elements.
IP High Court stated, “In this case, the essential elements should be decided by determining the characteristics (in the claims) consisting of the special technical ideas which were not shown in the prior arts after understanding the problems, the solutions and the effects of the patented invention based on the claims and the specification.”
“In addition, the essential elements should be decided by the description in the claims and the specification, especially the comparison between the specification and the prior arts. Moreover, the essential elements should be decided to (i) the generic concept of the parts of the claims if the contribution from the prior arts is great, (ii) almost the same as the claims if the contribution from the prior arts is not great.”
IP High Court also stated, “This invention can make it possible to manufacture the targeted substance by new route which was not shown in the prior arts. Therefore, it was a great contribution.” Then, it was decided that this new route is the essential element, and the cis-isomer (not the trans-isomer) as the structure of Vitamin D is non-essential element. Therefore, it was judged that the first requirement for the equivalency is satisfied.
(2) Fifth requirement for the equivalency (File Wrapper Estoppel)
Fifth requirement for the equivalency under the “Ballspline case” requires that there is no special circumstances to have intentionally excluded the products at issue from the claims during the filing procedures.
The fact that the element is not included in the claim is not necessary the special circumstance under the fifth requirement only by the reason for the case; “There is the element which is essentially the same as the claimed invention, and which could be easily reached by the persons ordinarily skilled in the field of the invention. This applicant could also reach this element.”
However, even in such case above, when it is objectively and superficially understandable that the applicant was understanding the element outside the claim as exchangeable one to the other element inside the claim (for example, when it can be considered that an applicant describes the invention of the other element in the specification, or when the invention of other element outside the claim is described in the articles by this applicant around the filing date), it is deemed to the special circumstance under the fifth requirement.
In this case, there is no description related to the vitamin D structure of trans-isomer in the specification, and there is no evidences objectively and superficially that this applicant was understanding the vitamin D structure of trans-isomer. Then, it was judged that there is no special circumstances under the fifth requirement.
<Comments>
This is the first case which affirmed the infringement by the reason for the equivalency to the claimed invention in pharmaceutical field.
The generic companies basically manufacture the generic medicines outside the claims. This judgment suggests that it is desirable for the generic companies to try to avoid the infringement more carefully with considering the infringement based on the equivalency as well as the literally infringement.
On the other hand, by this judgment, the possibility to the infringement based on the equivalency, namely more perfect protection was shown, which would push the promotion of the innovation that is expected in the originator pharmaceutical companies.
Note that IP High Court decided that vitamin D structure of trans-isomer is equivalent to that of cis-isomer under the fifth requirements, and equivalence infringement was affirmed.
The accumulation of the precedents is necessary to foresee the future direction. The future cases related to the infringement based on the equivalency would be carefully checked.
http://www.ip.courts.go.jp/app/files/hanrei_jp/769/085769_hanrei.pdf
Note
Five Requirements for Equivalency
by Supreme Court Decision “Ballspline case”
(February 24, 1998; H6 (O) 1083)
Even if there are the differences between claimed inventions and products (or methods) at issue, the products at issue are equivalent to the claimed inventions in case that all the five requirements are satisfied as follows;
<First requirement>
The differences are not the essential elements.
<Second requirement>
The same objective can be achieved if the differences are exchanged into the products at issue.
<Third requirement>
The differences can be easily exchanged into the products at issue by the persons ordinarily skilled in the field of the invention.
<Fourth requirement>
The products at issue is not identical to the publicly known technology at the time of the filing of this patent, and could not be easily invented from the publicly known technology at the time of the filing of this patent by the persons ordinarily skilled in the field of the invention.
<Fifth requirement>
There is no special circumstances to have intentionally excluded the products at issue from the claims during the filing procedures.

