IP High Court Case Summary: 2016 (Ne) 10031:
CLAIM LANGUAGE “BUFFERING AGENT” CONSTRUED NARROWLY IN LIGHT OF SPECIFICATION
On December 8, 2016, Intellectual Property High Court (IPHC) narrowly construed a claim language “buffering” and overturned the Tokyo District Court’s infringement decision (2015 (Wa) 12416).
BACKGROUND
Plaintiff-Appellee, Debiopharm International SA (“Debiopharm”) is an owner of patent (JP 4430229 B) claiming “Oxaliplatin solution formulations, preparation of such formations, and method of use thereof.” Defendant-Appellant, Nippon Kayaku Co.,Ltd. (“Nippon Kayaku”) manufactured and sold oxaliplatin formulation. Debiopharm alleged that the Nippon Kayaku’s product falls within the scope of the claimed invention and asked for an injunction to prevent the continuation of the manufacturing and selling the product. Tokyo District Court found in favor of Debiopharm and ruled that the claim language “oxalic acid” as the buffering agent encompasses both “added oxalic acid” and “dissociate oxalic acid” and the Nippon Kayaku’s product infringes the patent. Nippon Kayaku appealed to IPHC.
CLAIM
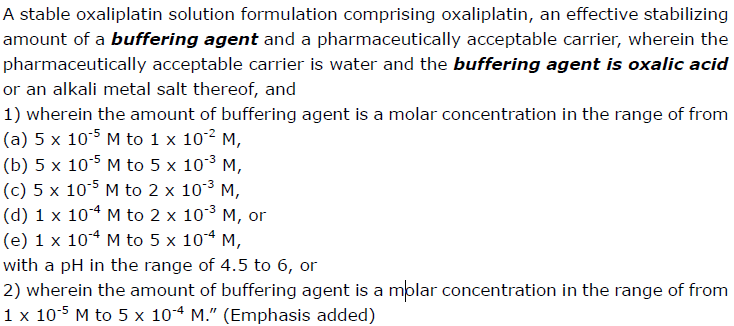
Claim 1 at issue reads:
NIPPON KAYAKU’S PRODUCTS
Nippon Kayaku’s product is oxaliplatin solution formulation, comprising oxaliplatin and water, the molar concentration of oxalic acid as buffering agent in the product ranging from 6.4×10-5 to 7.0×10-5M, and the pH being in the range of 3 to 4.5. The oxalic acid is “not added.”
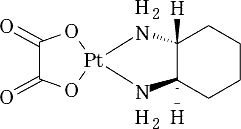
[Oxaliplatin]
(Source: PMDA website)
POINT AT ISSUE
At issue is whether, inter alia, oxalic acid as the buffering agent in claim 1 is limited to that added in oxaliplatin solution (“added oxalic acid”), disclosed in the specification, or encompasses not only added oxalic acid but also that generated by dissociation of oxaliplatin in the solution (“dissociated oxalic acid”), not disclosed in the specification. Tokyo district court construed claimed oxalic acid as the buffering agent so broadly that the Nippon Kayaku’s product including the dissociated oxalic acid falls within the scope of the invention.
IPHC’S DECISION
IPHC reversed the Tokyo District Court’s decision and ruled that claimed oxalic acid as the buffering agent should be limited to added oxalic acid, from the reasons below:
(a) Claimed Invention
The claimed invention relates to “the oxaliplatin solution composition” comprising oxaliplatin, buffering agent (oxalic acid) and water. Naturally, it should be understood that each of those elements exists independently in the composition. However, in the Nippon Kayaku’s product, the dissociated oxalic acid is generated by the reaction between oxaliplatin and water and thereby to disintegrate the oxaliplatin in the oxaliplatin solution composition. This means that the dissociated oxalic acid could exist only in a condition where oxaliplatin and water are mixed with each other. Therefore, it is irrational to see that the dissociated oxalic acid composing the oxaliplatin solution composition is independent of oxaliplatin and water.
(b) Common Technical Knowledge (Meaning of the Term)
Typically, “agent” is defined as one obtained by formulating various medicines or such medicine. According to this definition, the dissociated oxalic acid is not the buffering agent because it is not supposed to be formulated.
(c) Disclosures in Specification
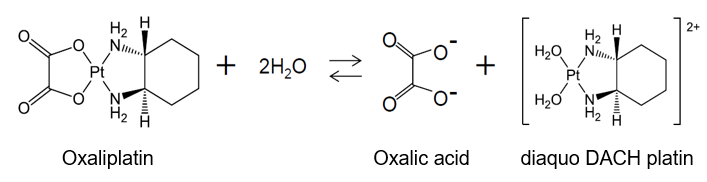
Regarding “buffering agent”, the patent specification recites “The term of the buffering agent as used herein means any acidic or basic agent which is capable of stabilizing oxaliplatin solutions and thereby preventing or retarding the generation of unwanted impurities such as diaquo DACH platin and diaquo DACH platin dimer.” In the meantime, dissociated oxalic acid is generated together with diaquo DACH platin by a partial degradation of the oxaliplatin in water solution; namely, dissociated oxalic acid is one of the components that form an equilibrium condition naturally caused by the reaction between oxaliplatin and water. Therefore, it is irrational to see the dissociated oxalic acid as a material that inhibit or retard the generation of substance such as diaquo DACH platin in the reaction toward the equilibrium condition.
(d) Description of Embodiments
The specification describes embodiments in each of which only the added buffering is used. Also, tables of ingredients for the embodiments show only weights and molar concentrations of oxalic acid or oxalic sodium added in the manufacturing process. As above, regarding the amount or molar concentration of the buffering agent, no description could be found in the specification that suggests a possible usage of the dissociated oxalic acid, and only the amount or molar concentration of the added oxalic acid is discussed in the specification.
Comments
This is the case in which construction of claim language is disputed. In this decision, the technical scope of the patented invention was determined based on the claim while the buffering agent was construed in light of descriptions of the specification, according to the Japanese Patent Act §70 (1) and (2). In particular, in the discussion whether “oxalic acid” acting as buffering agent is limited to the “added oxalic acid” or encompasses both “added oxalic acid” and “dissociated oxalic acid”, IPHC finally decided that “oxalic acid” as the buffering agent should be limited to the “added oxalic acid”, other than “dissociated oxalic acid”, primarily in light of the descriptions of the specification, as descried above.
The decision suggests that generic medicines of oxaliplatin can avoid patent infringement simply by not “adding” oxalic acid, which in turn teaches that claims should be drafted to ensure a suitable scope covering possible generic drugs.
Currently, invalidity of this patent is being discussed in IPHC (2015 (Gyo-ke) 10167), and a lot of attention has been paid to whether the same approach is applied in determining the gist of the invention in this litigation.
http://www.ip.courts.go.jp/app/files/hanrei_jp/325/086325_hanrei.pdf

